Lipidica is conducting a study on the functional capability of a lipidomic test titled ” Clinical performance of medical device software “Lipidica 1.0” for processing data generated by lipidomic analysis in pancreatic cancer screening” in collaboration with the research infrastructure CZECRIN, which operates at the Faculty of Medicine, Masaryk University, and in selected healthcare facilities. The key partners are the Masaryk Memorial Cancer Institute and the University Hospital Olomouc, where national pilot programs for pancreatic cancer screening in high-risk individuals are being conducted within the SCREPAN and HEPACAS studies.
The study was approved by State Institute for Drug Control (Czech regulatory authority) and Ethics Committees of participating hospitals.
Lipidica study is conducted under the auspices of the Czech Gastroenterological Society in the pancreas surveillance setting and is registered on clinicaltrials.gov. – ID: NCT06549725. The pilot study was published in the journal Nature Communication in the article Wolrab, D., Jirásko, R., Cífková, E. et al.: Lipidomic profiling of human serum enables detection of pancreatic cancer. Nat Commun 13, 124 (2022).

Enter the study by first checking your risk of pancreatic cancer using
Who can participate in the study?
For a statistically conclusive validation of the method, approx. 400 study participants will need to be recruited from the following groups, predominantly from the at-risk group:
- Newly diagnosed PDAC patients older than 18 years with stage 1 and 2 tumors (approximately 10% of newly diagnosed patients in the Czech Republic).
- Individuals at increased risk of developing pancreatic cancer (> 5%) (see Pancreatic cancer).
What benefits will participants gain from the study?
Individuals at increased risk of developing pancreatic cancer will benefit from regular preventive examinations, allowing for early diagnosis of tumors and precancerous conditions in the pancreas.
Additionally, the participation of each volunteer can help future patients achieve early disease diagnosis.
Where will the study take place?
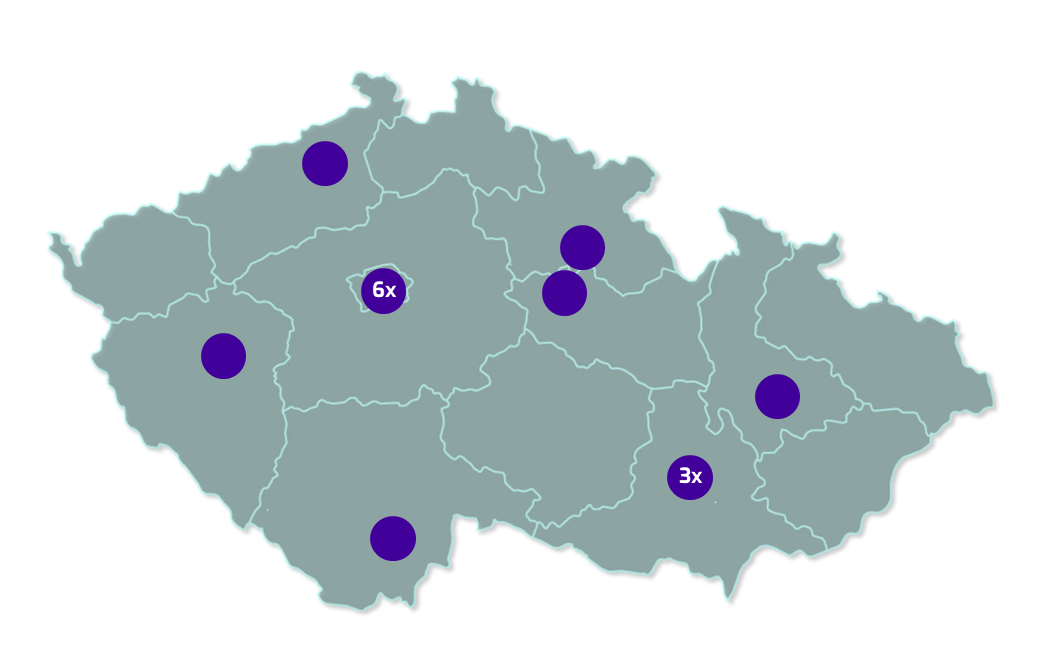
The study is being conducted in major hospitals across the Czech Republic. Currently, the individual centers are gradually opening for recruitment.
Map of hospitals involved in the study by region
How long will participants be enrolled in the study?
Patients with a diagnosed pancreatic cancer will participate in the study for one day.
Individuals at increased risk will participate in the study for 12 months ± 3 months. After completing their participation in the Lipidica study, participants may continue to be monitored in the pancreatic cancer screening programs Screpan or Hepacas.
How will the lipidomic test be conducted?
The anonymized (blinded) samples will be transported to the Lipidica laboratory, which will perform the LDPC lipidomic test. The results will be sent to a secure data repository and continuously evaluated with respect to the study objectives of assessing clinical function and determining sensitivity and specificity. The treating physician will receive the LDPC test results no later than three months after the sample is taken.
What other examinations will participants undergo during the study?
Patients with a diagnosed cancer will have one blood sample taken for the determination of the lipidomic test, oncological markers, and glycosylated hemoglobin.
Individuals at risk will undergo imaging examinations (EUS, CT, or MR) and a blood sample collection for the determination of the lipidomic test, oncological markers, and glycosylated hemoglobin at the beginning and end of the study. If the results from the first visit are not unequivocally negative from the lipidomic test and imaging methods, they will attend a visit after 6 months ± 3 months.
Participation in the study

Enter the study by first checking your risk of pancreatic cancer using
